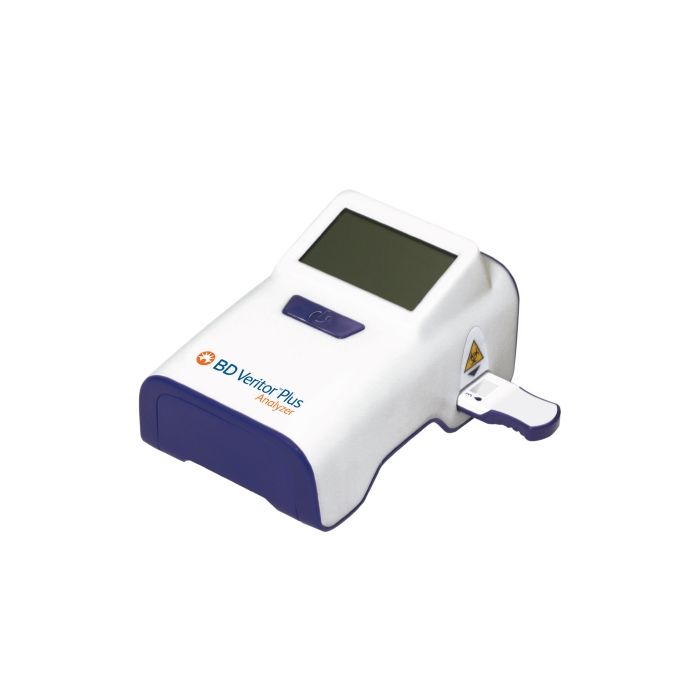
Veritor™ InfoScan Module 1 for Veritor™ Plus Analyzer
Non-Stock product. This product is not regularly stocked in our Warehouse.
Non-returnable.
- Veritor™ InfoScan Module 1 for Veritor™ Plus Analyzer
- Dimension: 7 L x 5 W x 3 in D
- Time To Results: 5 to 10 Minute Results
- Power Source: 100 to 240 VAC at 50 to 60 Hz, 0.6 A
- Sample Type: Throat Swab / Nasal Swab / Nasopharyngeal Swab / Nasopharyngeal Aspirate or Wash Sample
- Test Name: Influenza A + B, Respiratory Syncytial Virus (RSV), and Group A Strep
- Readout Type: Digital Readout, Printout (Option)
- User Interface: LCD Display
- CLIA Classification: CLIA Waived
- Contents 1: Module Only
- Compatible With: Veritor™ Plus Analyzer
- Weight: 350 g
- BD Veritor™ InfoScan Module is designed to add exceptional flexibility to your Veritor™ Plus Analyzer. It is intended to help customize your workflow, making data recording more efficient, less time consuming and error free. Streamlined Workflow - With BD Veritor™ InfoScan Module you are now able to revolutionize infectious disease testing and diagnostic right there at the point-of-care scene taking your data management and record keeping to a new level. Attached to your Veritor™ Plus Analyzer (256066), this Module facilitates traceability allowing local data storage and downloads. It helps you work faster reducing hands-on time throughout the testing process by providing an automated documentation function. As a result, the procedure data such as user ID, specimen ID, and test kit / lot can be printed, stored or downloaded eliminating manual record keeping errors and enhancing quality control process.